| Drug Name | IVW-1001 |
| Description | IVW-1001, a novel water-soluble TRPM8 agonist, is a promising candidate for relief of dry eye discomfort. It increases the tear secretion and improves pain symptom by acting on the TRPM8, a member of the TRP family of cation channel proteins. In an investigator sponsored clinical trial, IVW-1001 could increase secretion of tears within 5-20 minutes after the administration, with effects lasting longer after 2 weeks of administration. This benefit was not accompanied by any adverse events. In 2024, the FDA cleared the IND application for the initiation of a phase 1/2 clinical trial of IVW-1001 for the treatment of dry eye disease. |
| Target | TRPM8 |
| Drug Modality | Small molecule |
| Indication | Dry eye disease |
| Product Category | Signal transduction modulator |
| Mechanism of Action | Activating TRPM8 |
| Status | IND |
| Patent | Granted |
Protheragen Inc. is actively seeking partnership for IVW-1001. Potential collaboration can be strategic alliance, licensing, or marketing agreement.
We look forward to hearing from you.
| Introduction |
Transient receptor potential cation channel subfamily M member 8 (TRPM8) is a member of the melastatin-related TRP channel subfamily. It conducts Na+ and Ca2+ ions across cell membranes and is cold- and menthol-activated, which occurs via voltage sensing of membrane potential, phosphatidylinositol 4,5-bisphosphate and Ca2+. TRPM8 is expressed on sensory neurons in the cornea and eyelid, where it is associated with thermosensation. TRPM8 receptors are differentially expressed in the corneal epithelium, stroma and endothelium, where they are activated upon evaporation of the tear film. Abnormal TRPM8 reactivity may be one mechanism underlying the pathophysiology of dry eye disease, supporting the potential use of TRPM8 agonists to treat symptoms of ocular surface inflammation and pain in people with the disorder. |
| Approved Name | Transient receptor potential cation channel subfamily M member 8 |
| Official Symbol | TRPM8 |
| Gene Type | Protein coding |
| Synonyms | TRPP8; LTRPC6; trp-p8; LTrpC-6 |
| Ensembl | ENSG00000144481 |
| Gene ID | 79054 |
| mRNA Refseq | NM_024080 |
| Protein Refseq | NP_076985 |
| OMIM | 606678 |
| UniProt ID | Q7Z2W7 |
| Chromosome Location | 2q37.1 |
IVW-1001 is a new chemical entity called cryosim-3, which is a water-soluble TRPM8 receptor agonist. IVW-1001 has high selectivity for TRPM8 and has an optimal duration of drug action on the ocular surface. In a clinical trial, topical administration of IVW-1001 could relieve dry eye discomfort without any adverse effects.
Dry eye disease is a multifactorial disease characterized by a persistently unstable and/or deficient tear film causing discomfort and/or visual impairment, accompanied by variable degrees of ocular surface epitheliopathy, inflammation, and neurosensory abnormalities. Symptoms of dry eye disease include dryness, photosensitivity, blurred vision, redness, grittiness, foreign body sensation, pain, stinging, ocular fatigue, scratchiness, itchiness, sandiness, and soreness. Dry eye disease is classified as aqueous-deficient dry eye (ADDE), evaporative dry eye (EDE), or a combination of the two. The evaporative form is more common than the aqueous-deficient form, and mixed EDE/ADDE forms account for more than 80% of cases.
The prevalence of dry eye disease significantly increases with age, and women are more likely to develop dry eye than men. Worldwide, more than 11% of the population is thought to suffer from dry eye. Symptoms of dry eye disease have been reported in approximately one out of seven individuals above the age of 48, with its prevalence nearly doubling after 59 years of age. Treatment can reduce symptoms and prevent eye damage, but dry eye is a chronic condition for many patients, especially the elderly. There are relatively few effective treatments for dry eye disease. The most common treatments of dry eye disease include the use of lubricating eye drops, topical or oral anti-inflammatory drugs, as well as modification of diet and lifestyle.
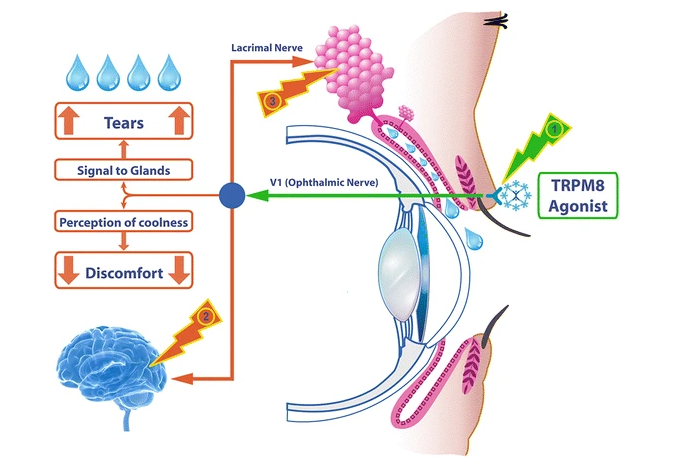
TRPM8 is a cold-sensing receptor (cold thermoreceptor) located on the nerve endings of the ophthalmic branch of the trigeminal nerve. The receptor regulates lacrimal function by response to evaporative cooling, cooling agents, and hypertonic stimulation. In addition, activation of TRPM8 may cause the central synaptic release of glutamate, which then suppresses injury-activated nociceptive afferent neurotransmission via inhibitory receptors in nerve endings. TRPM8 is distributed in the cornea and the eyelid. Therefore, TRPM8 can be activated by applying the agonist topically onto the eyelid, which increases basal tear secretion and reduces ocular discomfort through neuronal action, but with no direct effect on the tear film. IVW-1001 as a novel TRPM8 agonist has the potential to stimulate tear secretion and relieve neuropathic ocular pain in dry eye.
 Yang et al. BMC Ophthalmology (2017) 17:101. DOI 10.1186/s12886-017-0495-2
Yang et al. BMC Ophthalmology (2017) 17:101. DOI 10.1186/s12886-017-0495-2
In 2024, FDA cleared the investigational new drug (IND) application for the initiation of a phase 1/2 clinical trial as safe to proceed. The trial will evaluate the safety, tolerability, and efficacy of IVW-1001 to treat dry eye disease.
